University of Miami Researcher Hails Possible New AIDS Vaccine
The Florida Department of Health revealed some grim statistics on September 8: one in every 123 men in the state is living with HIV or AIDS, according to their latest study. The information, compiled between 1999 and 2008, also indicates that Miami-Dade leads the state in the number of Black and Hispanic men living with the disease. Here, one in every 29 Black men and one in every 82 Hispanic men is infected with the virus that causes AIDS. Despite the sober findings, recent developments at home and abroad have given afflicted individuals a new cause for optimism. THAI BREAKTHROUGH
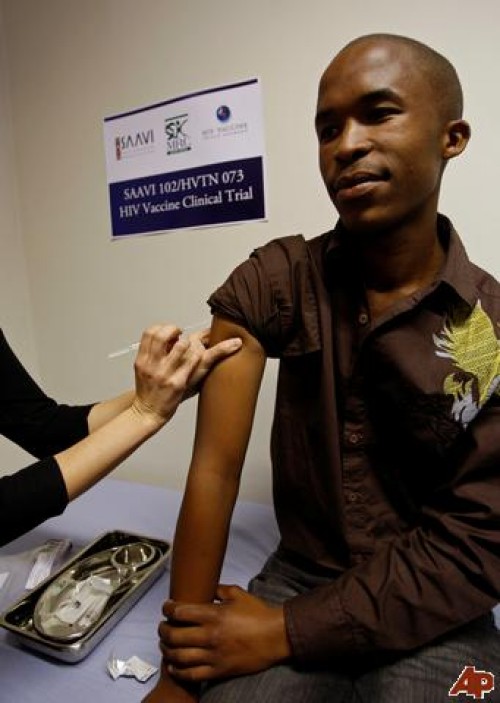
For the first time ever, researchers in Thailand have been able to prevent HIV infection in previously unexposed individuals at a success rate of nearly one third in trials funded by researchers from the US Army. More than 16,000 heterosexual men and women participated in the six-year clinical trial. Dr. Supachai Rerks-Ngarm of Thailand’s Ministry of Public Health stated that 74 who received placebos became infected by HIV, compared with 51 taking the vaccine. The vaccine was made from a modified smallpox vaccine developed by Sanofi Pasteur, together with a drug made from a synthetic version of a protein found in the AIDS virus. While the drug offers new hope to AIDS patients, it is only useful in patients who have not yet been exposed to HIV, the virus that causes AIDS. That’s where Dr. Margaret Fischl of the University of Miami comes in. FISCHL’S VACCINE
Dr. Margaret Fischl is a pioneering AIDS researcher at the University of Miami Medical School, who, in 1987, was among the principal developers of AZT, the first antiviral medicine to stop the AIDS virus from killing nearly everyone who became infected with the disease. Her new vaccine would be administered to patients already afflicted with the disease, helping to boost their immune systems. Up until now, it has been used successfully in small mammals up to the size of Rhesus monkeys, but by January, it will be ready to begin human trials. In a September 24 Miami Herald article, Fischl said, “The goal is to use the vaccine as the mainstay of treatment, so infected people would no longer need HAART (highly active antiretroviral therapy), with its expense and side effects. With this, they would take a shot every year to boost their systems and keep them in shape.” With this new treatment, patients currently using the so-called “cocktail” drug treatments would be able to take only one drug, drastically reducing the expense and side-effects associated with the present treatment. CAUTIOUS OPTIMISM
Dr. Fischl noted that even with volunteers lined around the block, it’s necessary to move slowly, starting with one patient and closely monitoring for side effects before moving on to the next one. “Human trials are very sensitive,” she observed, explaining that any enthusiasm must be tempered with an abundance of caution. Even assuming all goes well, the Thai vaccine and Dr. Fischl’s are both still a few years away. If the human trials are successful, doctors would begin replacing the medication regimes of those currently taking the cocktail with Fischl’s vaccine. Then, if results continued to be favorable, makers of the vaccine would seek fast-track approval by the FDA and it could be available to the general public in as little as three years. MIAMI ON THE CUTTING EDGE
It is encouraging that a region with some of the country’s highest AIDS rates may now be leading the way to a successful vaccine. We congratulate Dr. Fischl on her breakthroughs and eagerly anticipate seeing the results of her upcoming trials. Thanks in large part to her efforts, the worst years of this crisis may now be behind us.
You Deserve More Than an Ordinary Vacation.
Travel with Miami Beach 411 Today!
The Miami Beach 411 Travel Store is Open 24/7.
5 Comments on"University of Miami Researcher Hails Possible New AIDS Vaccine"
|

Like what you see? Let's talk about how
we can help your vacation --> Contact Us |
|
Like what you see? Let's talk about
how we can help your vacation
--> Contact Us
how we can help your vacation
--> Contact Us

















jungle man says:
This breakthrough will pose a serious treat to the pharmaceutical company even if will save millions of life’s. I will be surprise if the FDA approve this drug
Posted on 10/06/2009 at 9:34 PM